The FDA’s First Over-the-Counter Birth Control Approval Is a Game-Changer for Reproductive Rights
The Food and Drug Administration’s (FDA) approval of the first over-the-counter birth control is a huge victory for reproductive rights at a time when women need it the most. Birth control has been a game-changer ever since it was first legalized in the United States in 1965 and gave millions of women their first taste of reproductive freedom. Since then, further strides have been made in destigmatizing the pill and including it in coverage under health insurance plans. However, one roadblock that has remained to women’s access to birth control is the fact that it requires a prescription.
For years, it has been unclear why birth control requires a prescription. Yes, it does come with side effects, but these are rare and not much different from the typical risks associated with other over-the-counter medicines. Additionally, the risks associated with pregnancy seem to far outweigh the risks associated with birth control.
The problem with prescription birth control is it’s inconvenient and less easily accessible for people who need it, who sometimes may face months-long wait times to see a gynecologist or don’t have health insurance or the resources for a doctor’s visit, plus any follow-up appointments necessary to renew a prescription even once the initial one is secured. Healthcare companies like Nurx have made the process a little easier by allowing women to access and receive birth control online. However, that doesn’t eliminate every obstacle for people who may be in situations where they need a more discreet means to secure birth control. With the recent overturning of Roe v. Wade, it is even more urgent that the accessibility issues of prescription birth control are resolved.
Fortunately, access to contraceptives may improve significantly after the FDA’s approval of an over-the-counter birth control bill.
The FDA approves nonprescription birth control pill
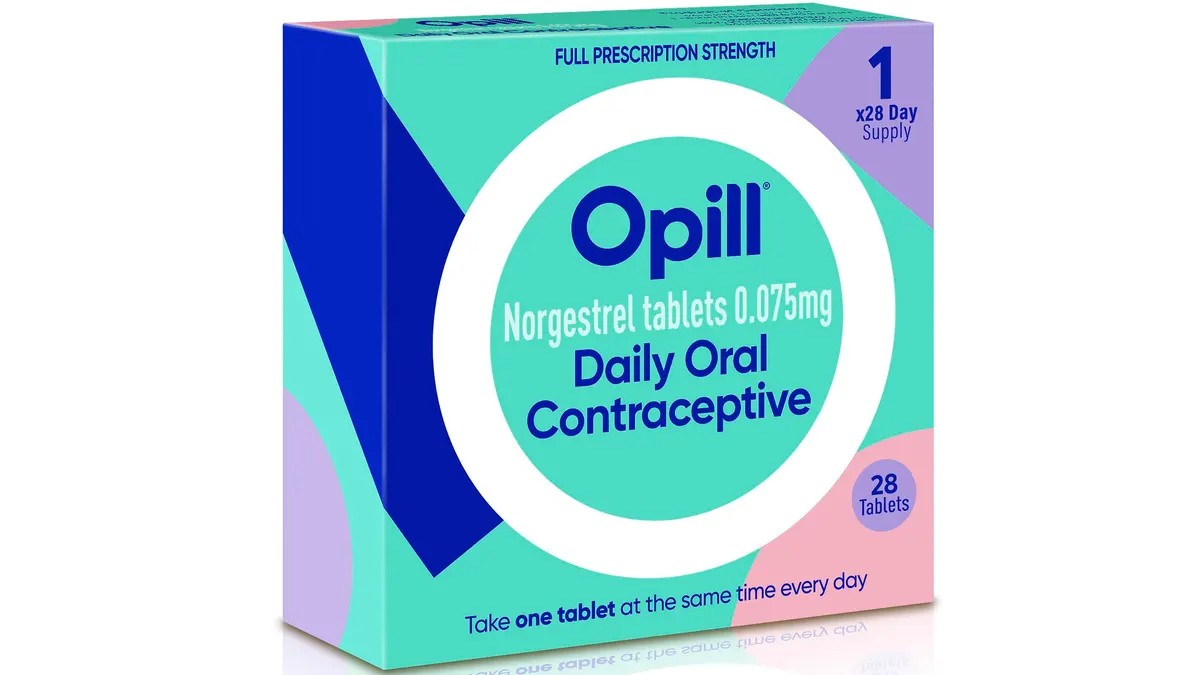
On July 13, the FDA approved the first-ever nonprescription birth control pill in the United States. The contraceptive is called Opill and is manufactured by the Dublin-based Perrigo Company, which hopes to have the pill available in the U.S. by early 2024. The pill is made with the hormone progestin, with the FDA emphasizing that it is safe and will be much more effective than current nonprescription contraceptive resources like condoms. With studies showing that access to reproductive health care is becoming more difficult, the FDA expressed hope that Opill could create “one more avenue in which people can access the care when they want it and how they want it.”
The decision comes after the FDA faced recommendations and pressure from advisers, lawmakers, and healthcare providers to approve Opill. There are a few risks associated with Opill, as the contraceptive is not safe for women with a history of breast cancer. It will also come with the usual potential side effects such as “irregular bleeding, headaches, dizziness, nausea, increased appetite, abdominal pain, cramps or bloating.” However, progestin-only birth control is believed to be very safe and comes with no serious risks. Additionally, the FDA is choosing to trust consumers to understand the risks, practice safe usage, and decide for themselves what’s right for their bodies.
It is expected that Opill will aid in providing millions with access to contraceptives and in decreasing the number of unintended pregnancies and complications from unintended pregnancies. The only unknown that remains is cost. Perrigo has indicated that they are dedicated to ensuring that Opill is affordable for people of all ages. The FDA is working on ensuring that Opill will be covered under insurance, as well. If Perrigo and the FDA pull through on the affordability aspect, Opill could be the breakthrough in accessibility to contraceptives for women that is long overdue.
(featured image: Perrigo Co.)
Have a tip we should know? [email protected]