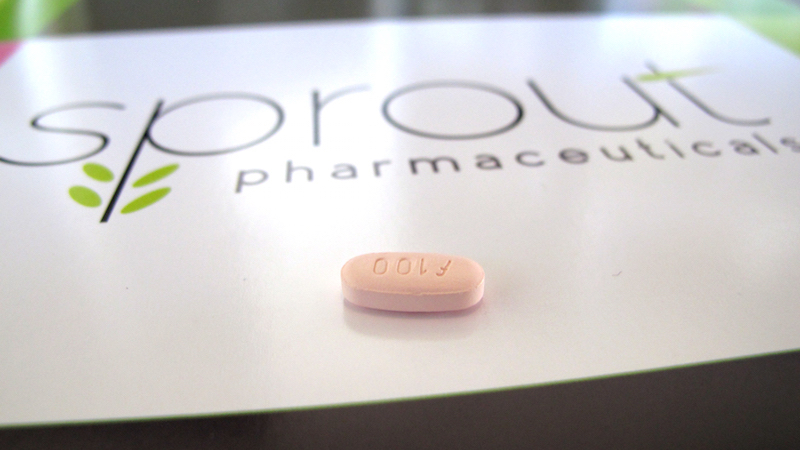
The FDA has officially approved a drug called flibanserin, to be marketed as “Addyi,” for the purpose of medicating sexual dysfunction in women. This drug will doubtless be referred to as “Lady Viagra” by everyone, but that’s quite a misnomer given how Addyi actually works. Here’s how NBC put it:
Unlike Viagra, which increases blood flow for men, Addyi works in a woman’s brain to solve a chemical imbalance among three neurotransmitters impacting desire. “We’re not trying to make people sex-crazed,” says Dr. Lauren Streicher of Northwestern University. “We’re trying to normalize what has been for them not only a very abnormal situation, but a very distressing situation.”
Dr. Streicher’s explanation struck me as a little odd — especially the “sex-crazed” bit. That’s not quite what Viagra does, and a real one-to-one equivalent to Viagra for women doesn’t sound like a bad idea to me. Some of my questions were answered by this article by sex educator Emily Nagoski, who doesn’t seem as thrilled about the potential power of Addyi. Here’s Nagoski drilling down the facts about how the drug worked when tested:
1. AT BEST 15% of the women who participated in the drug trial were “responders” — that is, their desire problems were “at least minimally improved,” above placebo — while about 85% were “non-responders,” whose desire problems were not even minimally improved by the drug.
2. On average, women on the drug had only one additional “sexually satisfying event” per month, above placebo.
One.
Remember, this is a drug you take every day… forever. So that you can have sex one more time per month.
Okay, so it doesn’t work for everybody. But if it helps some people, even a little bit, then that’s something … right? Not necessarily.
Negoski’s article got me to question whether Addyi medicates the right “problem” in the first place. So, Addyi works with neurotransmitters to make the patient feel more spontaneous about having sex. That’s cool and all, but should spontaneity be viewed as a medical necessity? Negoski writes,
A lot of us grew up learning the myth that desire is supposed to feel “spontaneous,” emerging before arousal. But the last twenty years of research have made it clear that, though many people can experience desire in anticipation of pleasure, other people, at least some of the time, experience desire in response to pleasure. Pleasure comes first, and desire emerges from it. It’s called “responsive desire,” and it’s normal.
…. Unfortunately only three of the twenty-four FDA panelists were sex researchers, therapists, or educators, so the panel believed the same old myth most of us were raised with — that the “urge” or “craving” is supposed to come first.
Nagoski concludes her article by advocating for couples therapy and sex therapy. Personally, I’m not convinced that’ll work for every patient, either. What about those patients whose sexual dysfunction isn’t psychosomatic?
Regardless, it does seem like having even more options beyond Addyi would be helpful in the future. If a lot of women want this drug, in spite of its relatively low efficacy, maybe their interest will bolster more support for future research.
(via Jezebel)
—Please make note of The Mary Sue’s general comment policy.—
Do you follow The Mary Sue on Twitter, Facebook, Tumblr, Pinterest, & Google +?







Published: Aug 19, 2015 11:40 am